Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. | The chemical reactions we have described are only a tiny sampling of the infinite number of chemical reactions possible. In this activity, students will learn about some of the ways to classify different types of chemical reactions. 3 nh3 + 3 n2 + 3 h2 b. This will include many synthesis and decomposition reactions. Check spelling or type a new query.
In layman language you can consider it like you swap your best friend with the other person's best friend. Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. Write a balanced equation for each of these chemical reactions. Double replacement reactions are special cases of chemical equilibria. Each of these reactions is referrred to as a synthesis reaction (sometimes.

Classify a chemical reaction as a synthesis, decomposition, single replacement, double replacement, or a combustion reaction. It requires two binary compounds, each of which exchanges one of its parts with the other. Substance p replaces x in the compound xy. In layman language you can consider it like you swap your best friend with the other person's best friend. Substance z burns in the presence of oxygen to form zo. Transcribed image text from this question. 3 nh3 + 3 n2 + 3 h2 b. Learn vocabulary, terms, and more with flashcards, games, and other study tools. In this activity, students will learn about some of the ways to classify different types of chemical reactions. The processes in which a substance or substances change to produce new substances with new properties are known as chemical reactions. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Decomposition reactions are really the opposite of combination reactions. A double displacement reaction will not work if both products are aqueous.
Give an example of each. Substance xyz breaks down into x, y, and z. Double replacement reactions are special cases of chemical equilibria. Salts like these tend to come apart into separate ions when placed in determine they type of each reaction below. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement.

This will include many synthesis and decomposition reactions. Substance xyz breaks down into x, y, and z. Substance x combines with y to form compound xy. Classifying chemical reactions worksheet from s3.studylib.net types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. In layman language you can consider it like you swap your best friend with the other person's best friend. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Check spelling or type a new query. Substitution and single replacement reactions. Which is a characteristic of a decomposition reaction? Chemical reactions the reaction in which a chemical substance transforms into another new types of decomposition reactions decomposition reactions can be classified into three types: Each of these reactions is referrred to as a synthesis reaction (sometimes. Predict the products of simple reactions. Decomposition reactions are really the opposite of combination reactions.
Substitution and single replacement reactions. Double displacement reaction or metathesis. Substance xyz breaks down into x, y, and z. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement.
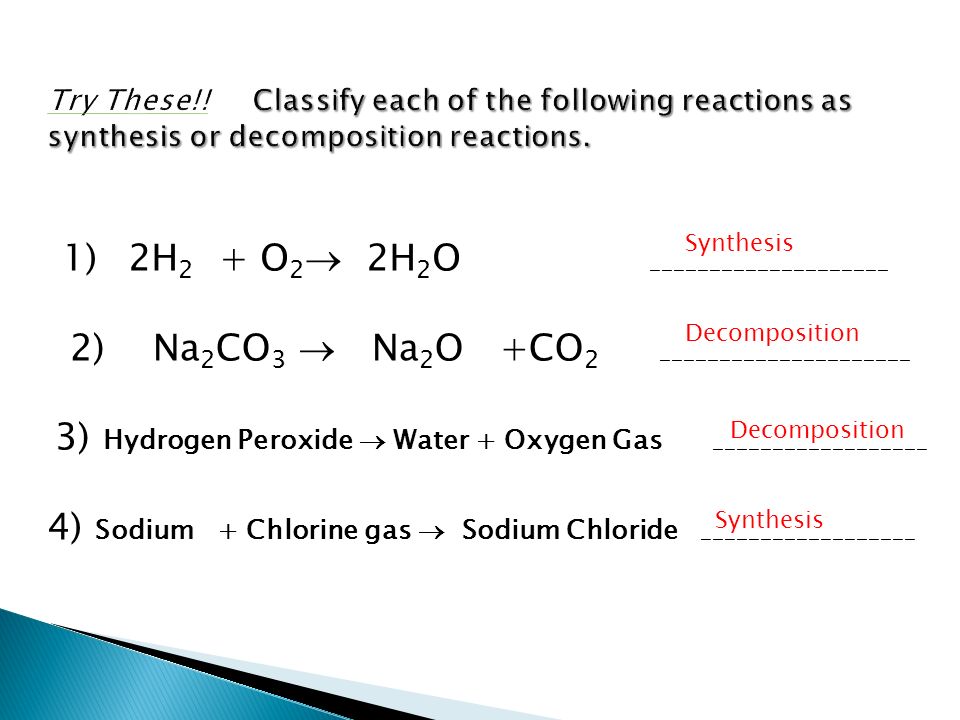
Classify each of these reactions. If you're still a bit shaky on how to tell the types of reactions apart or if you just want more examples, you can review the main reaction types. Substance z burns in the presence of oxygen to form zo. Substance x combines with y to form compound xy. The processes in which a substance or substances change to produce new substances with new properties are known as chemical reactions. Transcribed image text from this question. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. It requires two binary compounds, each of which exchanges one of its parts with the other. Beranda types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement. Each of these reactions is referrred to as a synthesis reaction (sometimes. In layman language you can consider it like you swap your best friend with the other person's best friend. 50 chemical reactions ideas chemical reactions teaching chemistry science chemistry. Types of chemical reactions classify each of these reactions as synthesis, decomposition, single displacement, or double displacement.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement.: Classify each of these reactions.
comment 0 Post a Comment
more_vert